- Chapter 9. Experimental studies

Randomised controlled trials
Crossover studies, experimental study of populations.
- Chapter 1. What is epidemiology?
- Chapter 2. Quantifying disease in populations
- Chapter 3. Comparing disease rates
- Chapter 4. Measurement error and bias
- Chapter 5. Planning and conducting a survey
- Chapter 6. Ecological studies
- Chapter 7. Longitudinal studies
- Chapter 8. Case-control and cross sectional studies
- Chapter 10. Screening
- Chapter 11. Outbreaks of disease
- Chapter 12. Reading epidemiological reports
- Chapter 13. Further reading
Follow us on
Content links.
- Collections
- Health in South Asia
- Women’s, children’s & adolescents’ health
- News and views
- BMJ Opinion
- Rapid responses
- Editorial staff
- BMJ in the USA
- BMJ in Latin America
- BMJ in South Asia
- Submit your paper
- BMA members
- Subscribers
- Advertisers and sponsors
Explore BMJ
- Our company
- BMJ Careers
- BMJ Learning
- BMJ Masterclasses
- BMJ Journals
- BMJ Student
- Academic edition of The BMJ
- BMJ Best Practice
- The BMJ Awards
- Email alerts
- Activate subscription
Information
- Call for Articles
- Login

Experimental Research Design — 6 mistakes you should never make!
Since school days’ students perform scientific experiments that provide results that define and prove the laws and theorems in science. These experiments are laid on a strong foundation of experimental research designs.
An experimental research design helps researchers execute their research objectives with more clarity and transparency.
In this article, we will not only discuss the key aspects of experimental research designs but also the issues to avoid and problems to resolve while designing your research study.
Table of Contents
What Is Experimental Research Design?
Experimental research design is a framework of protocols and procedures created to conduct experimental research with a scientific approach using two sets of variables. Herein, the first set of variables acts as a constant, used to measure the differences of the second set. The best example of experimental research methods is quantitative research .
Experimental research helps a researcher gather the necessary data for making better research decisions and determining the facts of a research study.
When Can a Researcher Conduct Experimental Research?
A researcher can conduct experimental research in the following situations —
- When time is an important factor in establishing a relationship between the cause and effect.
- When there is an invariable or never-changing behavior between the cause and effect.
- Finally, when the researcher wishes to understand the importance of the cause and effect.
Importance of Experimental Research Design
To publish significant results, choosing a quality research design forms the foundation to build the research study. Moreover, effective research design helps establish quality decision-making procedures, structures the research to lead to easier data analysis, and addresses the main research question. Therefore, it is essential to cater undivided attention and time to create an experimental research design before beginning the practical experiment.
By creating a research design, a researcher is also giving oneself time to organize the research, set up relevant boundaries for the study, and increase the reliability of the results. Through all these efforts, one could also avoid inconclusive results. If any part of the research design is flawed, it will reflect on the quality of the results derived.
Types of Experimental Research Designs
Based on the methods used to collect data in experimental studies, the experimental research designs are of three primary types:
1. Pre-experimental Research Design
A research study could conduct pre-experimental research design when a group or many groups are under observation after implementing factors of cause and effect of the research. The pre-experimental design will help researchers understand whether further investigation is necessary for the groups under observation.
Pre-experimental research is of three types —
- One-shot Case Study Research Design
- One-group Pretest-posttest Research Design
- Static-group Comparison
2. True Experimental Research Design
A true experimental research design relies on statistical analysis to prove or disprove a researcher’s hypothesis. It is one of the most accurate forms of research because it provides specific scientific evidence. Furthermore, out of all the types of experimental designs, only a true experimental design can establish a cause-effect relationship within a group. However, in a true experiment, a researcher must satisfy these three factors —
- There is a control group that is not subjected to changes and an experimental group that will experience the changed variables
- A variable that can be manipulated by the researcher
- Random distribution of the variables
This type of experimental research is commonly observed in the physical sciences.
3. Quasi-experimental Research Design
The word “Quasi” means similarity. A quasi-experimental design is similar to a true experimental design. However, the difference between the two is the assignment of the control group. In this research design, an independent variable is manipulated, but the participants of a group are not randomly assigned. This type of research design is used in field settings where random assignment is either irrelevant or not required.
The classification of the research subjects, conditions, or groups determines the type of research design to be used.
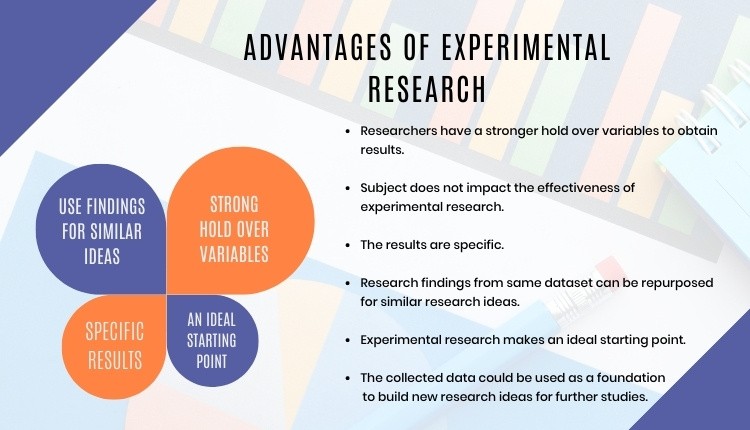
Advantages of Experimental Research
Experimental research allows you to test your idea in a controlled environment before taking the research to clinical trials. Moreover, it provides the best method to test your theory because of the following advantages:
- Researchers have firm control over variables to obtain results.
- The subject does not impact the effectiveness of experimental research. Anyone can implement it for research purposes.
- The results are specific.
- Post results analysis, research findings from the same dataset can be repurposed for similar research ideas.
- Researchers can identify the cause and effect of the hypothesis and further analyze this relationship to determine in-depth ideas.
- Experimental research makes an ideal starting point. The collected data could be used as a foundation to build new research ideas for further studies.
6 Mistakes to Avoid While Designing Your Research
There is no order to this list, and any one of these issues can seriously compromise the quality of your research. You could refer to the list as a checklist of what to avoid while designing your research.
1. Invalid Theoretical Framework
Usually, researchers miss out on checking if their hypothesis is logical to be tested. If your research design does not have basic assumptions or postulates, then it is fundamentally flawed and you need to rework on your research framework.
2. Inadequate Literature Study
Without a comprehensive research literature review , it is difficult to identify and fill the knowledge and information gaps. Furthermore, you need to clearly state how your research will contribute to the research field, either by adding value to the pertinent literature or challenging previous findings and assumptions.
3. Insufficient or Incorrect Statistical Analysis
Statistical results are one of the most trusted scientific evidence. The ultimate goal of a research experiment is to gain valid and sustainable evidence. Therefore, incorrect statistical analysis could affect the quality of any quantitative research.
4. Undefined Research Problem
This is one of the most basic aspects of research design. The research problem statement must be clear and to do that, you must set the framework for the development of research questions that address the core problems.
5. Research Limitations
Every study has some type of limitations . You should anticipate and incorporate those limitations into your conclusion, as well as the basic research design. Include a statement in your manuscript about any perceived limitations, and how you considered them while designing your experiment and drawing the conclusion.
6. Ethical Implications
The most important yet less talked about topic is the ethical issue. Your research design must include ways to minimize any risk for your participants and also address the research problem or question at hand. If you cannot manage the ethical norms along with your research study, your research objectives and validity could be questioned.
Experimental Research Design Example
In an experimental design, a researcher gathers plant samples and then randomly assigns half the samples to photosynthesize in sunlight and the other half to be kept in a dark box without sunlight, while controlling all the other variables (nutrients, water, soil, etc.)
By comparing their outcomes in biochemical tests, the researcher can confirm that the changes in the plants were due to the sunlight and not the other variables.
Experimental research is often the final form of a study conducted in the research process which is considered to provide conclusive and specific results. But it is not meant for every research. It involves a lot of resources, time, and money and is not easy to conduct, unless a foundation of research is built. Yet it is widely used in research institutes and commercial industries, for its most conclusive results in the scientific approach.
Have you worked on research designs? How was your experience creating an experimental design? What difficulties did you face? Do write to us or comment below and share your insights on experimental research designs!
Frequently Asked Questions
Randomization is important in an experimental research because it ensures unbiased results of the experiment. It also measures the cause-effect relationship on a particular group of interest.
Experimental research design lay the foundation of a research and structures the research to establish quality decision making process.
There are 3 types of experimental research designs. These are pre-experimental research design, true experimental research design, and quasi experimental research design.
The difference between an experimental and a quasi-experimental design are: 1. The assignment of the control group in quasi experimental research is non-random, unlike true experimental design, which is randomly assigned. 2. Experimental research group always has a control group; on the other hand, it may not be always present in quasi experimental research.
Experimental research establishes a cause-effect relationship by testing a theory or hypothesis using experimental groups or control variables. In contrast, descriptive research describes a study or a topic by defining the variables under it and answering the questions related to the same.
good and valuable
Very very good
Good presentation.
Rate this article Cancel Reply
Your email address will not be published.

Enago Academy's Most Popular Articles

- Promoting Research
Graphical Abstracts Vs. Infographics: Best practices for using visual illustrations for increased research impact
Dr. Sarah Chen stared at her computer screen, her eyes staring at her recently published…

- Publishing Research
10 Tips to Prevent Research Papers From Being Retracted
Research paper retractions represent a critical event in the scientific community. When a published article…

- Industry News
Google Releases 2024 Scholar Metrics, Evaluates Impact of Scholarly Articles
Google has released its 2024 Scholar Metrics, assessing scholarly articles from 2019 to 2023. This…
![experimental studies study What is Academic Integrity and How to Uphold it [FREE CHECKLIST]](https://www.enago.com/academy/wp-content/uploads/2024/05/FeatureImages-59-210x136.png)
Ensuring Academic Integrity and Transparency in Academic Research: A comprehensive checklist for researchers
Academic integrity is the foundation upon which the credibility and value of scientific findings are…

- Reporting Research
How to Optimize Your Research Process: A step-by-step guide
For researchers across disciplines, the path to uncovering novel findings and insights is often filled…
Choosing the Right Analytical Approach: Thematic analysis vs. content analysis for…
Comparing Cross Sectional and Longitudinal Studies: 5 steps for choosing the right…

Sign-up to read more
Subscribe for free to get unrestricted access to all our resources on research writing and academic publishing including:
- 2000+ blog articles
- 50+ Webinars
- 10+ Expert podcasts
- 50+ Infographics
- 10+ Checklists
- Research Guides
We hate spam too. We promise to protect your privacy and never spam you.
- Plagiarism Checker
- AI Content Detector
- Academic Editing
- Publication Support Services
- Thesis Editing
- Enago Report
- Journal Finder
- Diversity and Inclusion
- Al In Academia
- Career Corner
- Thought Leadership
- Other Resources
- Infographic
- Enago Learn
- On-Demand Webinar
- Open Access Week 2024
- Peer Review Week 2024
- Publication Integrity Week
- Conference Videos
- Call For speakers
- Author Training
I am looking for Editing/ Proofreading services for my manuscript Tentative date of next journal submission:

What features do you prefer in a plagiarism detector? (Select all that apply)
- Privacy Policy

Home » Experimental Design – Types, Methods, Guide
Experimental Design – Types, Methods, Guide
Table of Contents
Experimental design is a structured approach used to conduct scientific experiments. It enables researchers to explore cause-and-effect relationships by controlling variables and testing hypotheses. This guide explores the types of experimental designs, common methods, and best practices for planning and conducting experiments.

Experimental Design
Experimental design refers to the process of planning a study to test a hypothesis, where variables are manipulated to observe their effects on outcomes. By carefully controlling conditions, researchers can determine whether specific factors cause changes in a dependent variable.
Key Characteristics of Experimental Design :
- Manipulation of Variables : The researcher intentionally changes one or more independent variables.
- Control of Extraneous Factors : Other variables are kept constant to avoid interference.
- Randomization : Subjects are often randomly assigned to groups to reduce bias.
- Replication : Repeating the experiment or having multiple subjects helps verify results.
Purpose of Experimental Design
The primary purpose of experimental design is to establish causal relationships by controlling for extraneous factors and reducing bias. Experimental designs help:
- Test Hypotheses : Determine if there is a significant effect of independent variables on dependent variables.
- Control Confounding Variables : Minimize the impact of variables that could distort results.
- Generate Reproducible Results : Provide a structured approach that allows other researchers to replicate findings.
Types of Experimental Designs
Experimental designs can vary based on the number of variables, the assignment of participants, and the purpose of the experiment. Here are some common types:
1. Pre-Experimental Designs
These designs are exploratory and lack random assignment, often used when strict control is not feasible. They provide initial insights but are less rigorous in establishing causality.
- Example : A training program is provided, and participants’ knowledge is tested afterward, without a pretest.
- Example : A group is tested on reading skills, receives instruction, and is tested again to measure improvement.
2. True Experimental Designs
True experiments involve random assignment of participants to control or experimental groups, providing high levels of control over variables.
- Example : A new drug’s efficacy is tested with patients randomly assigned to receive the drug or a placebo.
- Example : Two groups are observed after one group receives a treatment, and the other receives no intervention.
3. Quasi-Experimental Designs
Quasi-experiments lack random assignment but still aim to determine causality by comparing groups or time periods. They are often used when randomization isn’t possible, such as in natural or field experiments.
- Example : Schools receive different curriculums, and students’ test scores are compared before and after implementation.
- Example : Traffic accident rates are recorded for a city before and after a new speed limit is enforced.
4. Factorial Designs
Factorial designs test the effects of multiple independent variables simultaneously. This design is useful for studying the interactions between variables.
- Example : Studying how caffeine (variable 1) and sleep deprivation (variable 2) affect memory performance.
- Example : An experiment studying the impact of age, gender, and education level on technology usage.
5. Repeated Measures Design
In repeated measures designs, the same participants are exposed to different conditions or treatments. This design is valuable for studying changes within subjects over time.
- Example : Measuring reaction time in participants before, during, and after caffeine consumption.
- Example : Testing two medications, with each participant receiving both but in a different sequence.
Methods for Implementing Experimental Designs
- Purpose : Ensures each participant has an equal chance of being assigned to any group, reducing selection bias.
- Method : Use random number generators or assignment software to allocate participants randomly.
- Purpose : Prevents participants or researchers from knowing which group (experimental or control) participants belong to, reducing bias.
- Method : Implement single-blind (participants unaware) or double-blind (both participants and researchers unaware) procedures.
- Purpose : Provides a baseline for comparison, showing what would happen without the intervention.
- Method : Include a group that does not receive the treatment but otherwise undergoes the same conditions.
- Purpose : Controls for order effects in repeated measures designs by varying the order of treatments.
- Method : Assign different sequences to participants, ensuring that each condition appears equally across orders.
- Purpose : Ensures reliability by repeating the experiment or including multiple participants within groups.
- Method : Increase sample size or repeat studies with different samples or in different settings.
Steps to Conduct an Experimental Design
- Clearly state what you intend to discover or prove through the experiment. A strong hypothesis guides the experiment’s design and variable selection.
- Independent Variable (IV) : The factor manipulated by the researcher (e.g., amount of sleep).
- Dependent Variable (DV) : The outcome measured (e.g., reaction time).
- Control Variables : Factors kept constant to prevent interference with results (e.g., time of day for testing).
- Choose a design type that aligns with your research question, hypothesis, and available resources. For example, an RCT for a medical study or a factorial design for complex interactions.
- Randomly assign participants to experimental or control groups. Ensure control groups are similar to experimental groups in all respects except for the treatment received.
- Randomize the assignment and, if possible, apply blinding to minimize potential bias.
- Follow a consistent procedure for each group, collecting data systematically. Record observations and manage any unexpected events or variables that may arise.
- Use appropriate statistical methods to test for significant differences between groups, such as t-tests, ANOVA, or regression analysis.
- Determine whether the results support your hypothesis and analyze any trends, patterns, or unexpected findings. Discuss possible limitations and implications of your results.
Examples of Experimental Design in Research
- Medicine : Testing a new drug’s effectiveness through a randomized controlled trial, where one group receives the drug and another receives a placebo.
- Psychology : Studying the effect of sleep deprivation on memory using a within-subject design, where participants are tested with different sleep conditions.
- Education : Comparing teaching methods in a quasi-experimental design by measuring students’ performance before and after implementing a new curriculum.
- Marketing : Using a factorial design to examine the effects of advertisement type and frequency on consumer purchase behavior.
- Environmental Science : Testing the impact of a pollution reduction policy through a time series design, recording pollution levels before and after implementation.
Experimental design is fundamental to conducting rigorous and reliable research, offering a systematic approach to exploring causal relationships. With various types of designs and methods, researchers can choose the most appropriate setup to answer their research questions effectively. By applying best practices, controlling variables, and selecting suitable statistical methods, experimental design supports meaningful insights across scientific, medical, and social research fields.
- Campbell, D. T., & Stanley, J. C. (1963). Experimental and Quasi-Experimental Designs for Research . Houghton Mifflin Company.
- Shadish, W. R., Cook, T. D., & Campbell, D. T. (2002). Experimental and Quasi-Experimental Designs for Generalized Causal Inference . Houghton Mifflin.
- Fisher, R. A. (1935). The Design of Experiments . Oliver and Boyd.
- Field, A. (2013). Discovering Statistics Using IBM SPSS Statistics . Sage Publications.
- Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences . Routledge.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Mixed Methods Research – Types & Analysis

Exploratory Research – Types, Methods and...

Research Methods – Types, Examples and Guide

Correlational Research – Methods, Types and...


Quasi-Experimental Research Design – Types...

Ethnographic Research -Types, Methods and Guide
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Observational and interventional study design types; an overview
Matthew s thiese.
- Author information
- Article notes
- Copyright and License information
Corresponding author: [email protected]
Received 2013 Oct 14; Accepted 2014 Apr 12; Collection date 2014 Jun.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc-nd/3.0/ ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The appropriate choice in study design is essential for the successful execution of biomedical and public health research. There are many study designs to choose from within two broad categories of observational and interventional studies. Each design has its own strengths and weaknesses, and the need to understand these limitations is necessary to arrive at correct study conclusions.
Observational study designs, also called epidemiologic study designs, are often retrospective and are used to assess potential causation in exposure-outcome relationships and therefore influence preventive methods. Observational study designs include ecological designs, cross sectional, case-control, case-crossover, retrospective and prospective cohorts. An important subset of observational studies is diagnostic study designs, which evaluate the accuracy of diagnostic procedures and tests as compared to other diagnostic measures. These include diagnostic accuracy designs, diagnostic cohort designs, and diagnostic randomized controlled trials.
Interventional studies are often prospective and are specifically tailored to evaluate direct impacts of treatment or preventive measures on disease. Each study design has specific outcome measures that rely on the type and quality of data utilized. Additionally, each study design has potential limitations that are more severe and need to be addressed in the design phase of the study. This manuscript is meant to provide an overview of study design types, strengths and weaknesses of common observational and interventional study designs.
Keywords: study design, epidemiology, observational study, randomized trials, study strengths and weaknesses
Introduction
Study design plays an important role in the quality, execution, and interpretation of biomedical and public health research ( 1 – 12 ). Each study design has their own inherent strengths and weaknesses, and there can be a general hierarchy in study designs, however, any hierarchy cannot be applied uniformly across study design types ( 3 , 5 , 6 , 9 ). Epidemiological and interventional research studies include three elements; 1) definition and measure of exposure in two or more groups, 2) measure of health outcome(s) in these same groups, and 3) statistical comparison made between groups to assess potential relationships between the exposure and outcome, all of which are defined by the researcher ( 1 – 4 , 8 , 13 ). The measure of exposure in epidemiologic studies may be tobacco use (“Yes” vs . “No”) to define the two groups and may be the treatment (Active drug vs . placebo) in interventional studies. Health outcome(s) can be the development of a disease or symptom (e.g. lung cancer) or curing a disease or symptom (e.g. reduction of pain). Descriptive studies, which are not epidemiological or interventional, lack one or more of these elements and have limited application. High quality epidemiological and interventional studies contain detailed information on the design, execution and interpretation of results, with methodology clearly written and able to be reproduced by other researchers.
Research is generally considered as primary or secondary research. Primary research relies upon data gathered from original research expressly for that purpose ( 1 , 3 , 5 ). Secondary research focuses on single or multiple data sources that are not collected for a single research purpose ( 14 , 15 ). Secondary research includes meta-analyses and best practice guidelines for treatments. This paper will focus on the study designs and their strengths, weaknesses, and common statistical outcomes of primary research.
The choice of a study design hinges on many factors, including prior research, availability of study participants, funding, and time constraints. One common decision point is the desire to suggest causation. The most common causation criteria are proposed by Hill ( 16 ). Of these, demonstrating temporality is the only mandatory criterion for suggesting temporality. Therefore, prospective studies that follow study participants forward through time, including prospective cohort studies and interventional studies, are best suited for suggesting causation. Causal conclusions cannot be proven from an observational study. Additionally, causation between an exposure and an outcome cannot be proven by one study alone; multiple studies across different populations should be considered when making causation assessments ( 17 ).
Primary research has been categorized in different ways. Common categorization schema include temporal nature of the study design (retrospective or prospective), usability of the study results (basic or applied), investigative purpose (descriptive or analytical), purpose (prevention, diagnosis or treatment), or role of the investigator (observational or interventional). This manuscript categorizes study designs by observational and interventional criteria, however, other categorization methods are described as well.
Observational and interventional studies
Within primary research there are observational studies and interventional studies. Observational studies, also called epidemiological studies, are those where the investigator is not acting upon study participants, but instead observing natural relationships between factors and outcomes. Diagnostic studies are classified as observational studies, but are a unique category and will be discussed independently. Interventional studies, also called experimental studies, are those where the researcher intercedes as part of the study design. Additionally, study designs may be classified by the role that time plays in the data collection, either retrospective or prospective. Retrospective studies are those where data are collected from the past, either through records created at that time or by asking participants to remember their exposures or outcomes. Retrospective studies cannot demonstrate temporality as easily and are more prone to different biases, particularly recall bias. Prospective studies follow participants forward through time, collecting data in the process. Prospective studies are less prone to some types of bias and can more easily demonstrate that the exposure preceded the disease, thereby more strongly suggesting causation. Table 1 describes the broad categories of observational studies: the disease measures applicable to each, the appropriate measures of risk, and temporality of each study design. Epidemiologic measures include point prevalence, the proportion of participants with disease at a given point in time, period prevalence, the proportion of participants with disease within a specified time frame, and incidence, the accumulation of new cases over time. Measures of risk are generally categorized into two categories: those that only demonstrate an association, such as an odds ratio (and some other measures), and those that demonstrate temporality and therefore suggest causation, such as hazard ratio. Table 2 outlines the strengths and weaknesses of each observational study design.
Observational study design measures of disease, measures of risk, and temporality.
Observational study design strengths and weaknesses.
Observational studies
Ecological study design.
The most basic observational study is an ecological study. This study design compares clusters of people, usually grouped based on their geographical location or temporal associations ( 1 , 2 , 6 , 9 ). Ecological studies assign one exposure level for each distinct group and can provide a rough estimation of prevalence of disease within a population. Ecological studies are generally retrospective. An example of an ecological study is the comparison of the prevalence of obesity in the United States and France. The geographic area is considered the exposure and the outcome is obesity. There are inherent potential weaknesses with this approach, including loss of data resolution and potential misclassification ( 10 , 11 , 13 , 18 , 19 ). This type of study design also has additional weaknesses. Typically these studies derive their data from large databases that are created for purposes other than research, which may introduce error or misclassification ( 10 , 11 ). Quantification of both the number of cases and the total population can be difficult, leading to error or bias. Lastly, due to the limited amount of data available, it is difficult to control for other factors that may mask or falsely suggest a relationship between the exposure and the outcome. However, ecological studies are generally very cost effective and are a starting point for hypothesis generation.
Proportional mortality ratio study design
Proportional mortality ratio studies (PMR) utilize the defined well recorded outcome of death and subsequent records that are maintained regarding the decedent ( 1 , 6 , 8 , 20 ). By using records, this study design is able to identify potential relationships between exposures, such as geographic location, occupation, or age and cause of death. The epidemiological outcomes of this study design are proportional mortality ratio and standardized mortality ratio. In general these are the ratio of the proportion of cause-specific deaths out of all deaths between exposure categories ( 20 ). As an example, these studies can address questions about higher proportion of cardiovascular deaths among different ethnic and racial groups ( 21 ). A significant drawback to the PMR study design is that these studies are limited to death as an outcome ( 3 , 5 , 22 ). Additionally, the reliance on death records makes it difficult to control for individual confounding factors, variables that either conceal or falsely demonstrate associations between the exposure and outcome. An example of a confounder is tobacco use confounding the relationship between coffee intake and cardiovascular disease. Historically people often smoked and drank coffee while on coffee breaks. If researchers ignore smoking they would inaccurately find a strong relationship between coffee use and cardiovascular disease, where some of the risk is actually due to smoking. There are also concerns regarding the accuracy of death certificate data. Strengths of the study design include the well-defined outcome of death, the relative ease and low cost of obtaining data, and the uniformity of collection of these data across different geographical areas.
Cross-sectional study design
Cross-sectional studies are also called prevalence studies because one of the main measures available is study population prevalence ( 1 – 12 ). These studies consist of assessing a population, as represented by the study sample, at a single point in time. A common cross-sectional study type is the diagnostic accuracy study, which is discussed later. Cross-sectional study samples are selected based on their exposure status, without regard for their outcome status. Outcome status is obtained after participants are enrolled. Ideally, a wider distribution of exposure will allow for a higher likelihood of finding an association between the exposure and outcome if one exists ( 1 – 3 , 5 , 8 ). Cross-sectional studies are retrospective in nature. An example of a cross-sectional study would be enrolling participants who are either current smokers or never smokers, and assessing whether or not they have respiratory deficiencies. Random sampling of the population being assessed is more important in cross-sectional studies as compared to other observational study designs. Selection bias from non-random sampling may result in flawed measure of prevalence and calculation of risk. The study sample is assessed for both exposure and outcome at a single point in time. Because both exposure and outcome are assessed at the same time, temporality cannot be demonstrated, i.e. it cannot be demonstrated that the exposure preceded the disease ( 1 – 3 , 5 , 8 ). Point prevalence and period prevalence can be calculated in cross-sectional studies. Measures of risk for the exposure-outcome relationship that can be calculated in cross-sectional study design are odds ratio, prevalence odds ratio, prevalence ratio, and prevalence difference. Cross-sectional studies are relatively inexpensive and have data collected on an individual which allows for more complete control for confounding. Additionally, cross-sectional studies allow for multiple outcomes to be assessed simultaneously.
Case-control study design
Case-control studies were traditionally referred to as retrospective studies, due to the nature of the study design and execution ( 1 – 12 , 23 , 24 ). In this study design, researchers identify study participants based on their case status, i.e. diseased or not diseased. Quantification of the number of individuals among the cases and the controls who are exposed allow for statistical associations between exposure and outcomes to be established ( 1 – 3 , 5 , 8 ). An example of a case control study is analysing the relationship between obesity and knee replacement surgery. Cases are participants who have had knee surgery, and controls are a random sampling of those who have not, and the comparison is the relative odds of being obese if you have knee surgery as compared to those that do not. Matching on one or more potential confounders allows for minimization of those factors as potential confounders in the exposure-outcome relationship ( 1 – 3 , 5 , 8 ). Additionally, case-control studies are at increased risk for bias, particularly recall bias, due to the known case status of study participants ( 1 – 3 , 5 , 8 ). Other points of consideration that have specific weight in case-control studies include the appropriate selection of controls that balance generalizability and minimize bias, the minimization of survivor bias, and the potential for length time bias ( 25 ). The largest strength of case-control studies is that this study design is the most efficient study design for rare diseases. Additional strengths include low cost, relatively fast execution compared to cohort studies, the ability to collect individual participant specific data, the ability to control for multiple confounders, and the ability to assess multiple exposures of interest. The measure of risk that is calculated in case-control studies is the odds ratio, which are the odds of having the exposure if you have the disease. Other measures of risk are not applicable to case-control studies. Any measure of prevalence and associated measures, such as prevalence odds ratio, in a case-control study is artificial because the researcher arbitrarily sets the proportion of cases to non-cases in this study design. Temporality can be suggested, however, it is rarely definitively demonstrated because it is unknown if the development of the disease truly preceded the exposure. It should be noted that for certain outcomes, particularly death, the criteria for demonstrating temporality in that specific exposure-outcome relationship are met and the use of relative risk as a measure of risk may be justified.
Case-crossover study design
A case-crossover study relies upon an individual to act as their own control for comparison issues, thereby minimizing some potential confounders ( 1 , 5 , 12 ). This study design should not be confused with a crossover study design which is an interventional study type and is described below. For case-crossover studies, cases are assessed for their exposure status immediately prior to the time they became a case, and then compared to their own exposure at a prior point where they didn’t become a case. The selection of the prior point for comparison issues is often chosen at random or relies upon a mean measure of exposure over time. Case-crossover studies are always retrospective. An example of a case-crossover study would be evaluating the exposure of talking on a cell phone and being involved in an automobile crash. Cases are drivers involved in a crash and the comparison is that same driver at a random timeframe where they were not involved in a crash. These types of studies are particularly good for exposure-outcome relationships where the outcome is acute and well defined, e.g. electrocutions, lacerations, automobile crashes, etc. ( 1 , 5 ). Exposure-outcome relationships that are assessed using case-crossover designs should have health outcomes that do not have a subclinical or undiagnosed period prior to becoming a “case” in the study ( 12 ). The exposure is cell phone use during the exposure periods, both before the crash and during the control period. Additionally, the reliance upon prior exposure time requires that the exposure not have an additive or cumulative effect over time ( 1 , 5 ). Case-crossover study designs are at higher risk for having recall bias as compared with other study designs ( 12 ). Study participants are more likely to remember an exposure prior to becoming a case, as compared to not becoming a case.
Retrospective and prospective cohort study design
Cohort studies involve identifying study participants based on their exposure status and either following them through time to identify which participants develop the outcome(s) of interest, or look back at data that were created in the past, prior to the development of the outcome. Prospective cohort studies are considered the gold standard of observational research ( 1 – 3 , 5 , 8 , 10 , 11 ). These studies begin with a cross-sectional study to categorize exposure and identify cases at baseline. Disease-free participants are then followed and cases are measured as they develop. Retrospective cohort studies also begin with a cross-sectional study to categorize exposure and identify cases. Exposures are then measured based on records created at that time. Additionally, in an ideal retrospective cohort, case status is also tracked using historical data that were created at that point in time. Occupational groups, particularly those that have regular surveillance or certifications such as Commercial Truck Drivers, are particularly well positioned for retrospective cohort studies because records of both exposure and outcome are created as part of commercial and regulatory purposes ( 8 ). These types of studies have the ability to demonstrate temporality and therefore identify true risk factors, not associated factors, as can be done in other types of studies.
Cohort studies are the only observational study that can calculate incidence, both cumulative incidence and an incidence rate ( 1 , 3 , 5 , 6 , 10 , 11 ). Also, because the inception of a cohort study is identical to a cross-sectional study, both point prevalence and period prevalence can be calculated. There are many measures of risk that can be calculated from cohort study data. Again, the measures of risk for the exposure-outcome relationship that can be calculated in cross-sectional study design of odds ratio, prevalence odds ratio, prevalence ratio, and prevalence difference can be calculated in cohort studies as well. Measures of risk that leverage a cohort study’s ability to calculate incidence include incidence rate ratio, relative risk, risk ratio, and hazard ratio. These measures that demonstrate temporality are considered stronger measures for demonstrating causation and identification of risk factors.
Diagnostic testing and evaluation study designs
A specific study design is the diagnostic accuracy study, which is often used as part of the clinical decision making process. Diagnostic accuracy study designs are those that compare a new diagnostic method with the current “gold standard” diagnostic procedure in a cross-section of both diseased and healthy study participants. Gold standard diagnostic procedures are the current best-practice for diagnosing a disease. An example is comparing a new rapid test for a cancer with the gold standard method of biopsy. There are many intricacies to diagnostic testing study designs that should be considered. The proper selection of the gold standard evaluation is important for defining the true measures of accuracy for the new diagnostic procedure. Evaluations of diagnostic test results should be blinded to the case status of the participant. Similar to the intention-to-treat concept discussed later in interventional studies, diagnostic tests have a procedure of analyses called intention to diagnose (ITD), where participants are analysed in the diagnostic category they were assigned, regardless of the process in which a diagnosis was obtained. Performing analyses according to an a priori defined protocol, called per protocol analyses (PP or PPA), is another potential strength to diagnostic study testing. Many measures of the new diagnostic procedure, including accuracy, sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio can be calculated. These measures of the diagnostic test allow for comparison with other diagnostic tests and aid the clinician in determining which test to utilize.
Interventional study designs
Interventional study designs, also called experimental study designs, are those where the researcher intervenes at some point throughout the study. The most common and strongest interventional study design is a randomized controlled trial, however, there are other interventional study designs, including pre-post study design, non-randomized controlled trials, and quasi-experiments ( 1 , 5 , 13 ). Experimental studies are used to evaluate study questions related to either therapeutic agents or prevention. Therapeutic agents can include prophylactic agents, treatments, surgical approaches, or diagnostic tests. Prevention can include changes to protective equipment, engineering controls, management, policy or any element that should be evaluated as to a potential cause of disease or injury.
Pre-post study design
A pre-post study measures the occurrence of an outcome before and again after a particular intervention is implemented. A good example is comparing deaths from motor vehicle crashes before and after the enforcement of a seat-belt law. Pre-post studies may be single arm, one group measured before the intervention and again after the intervention, or multiple arms, where there is a comparison between groups. Often there is an arm where there is no intervention. The no-intervention arm acts as the control group in a multi-arm pre-post study. These studies have the strength of temporality to be able to suggest that the outcome is impacted by the intervention, however, pre-post studies do not have control over other elements that are also changing at the same time as the intervention is implemented. Therefore, changes in disease occurrence during the study period cannot be fully attributed to the specific intervention. Outcomes measured for pre-post intervention studies may be binary health outcomes such as incidence or prevalence, or mean values of a continuous outcome such as systolic blood pressure may also be used. The analytic methods of pre-post studies depend on the outcome being measured. If there are multiple treatment arms, it is also likely that the difference from beginning to end within each treatment arm are analysed.
Non-randomized trial study design
Non-randomized trials are interventional study designs that compare a group where an intervention was performed with a group where there was no intervention. These are convenient study designs that are most often performed prospectively and can suggest possible relationships between the intervention and the outcome. However, these study designs are often subject to many types of bias and error and are not considered a strong study design.
Randomized controlled trial study design
Randomized controlled trials (RCTs) are the most common type of interventional study, and can have many modifications ( 26 – 28 ). These trials take a homogenous group of study participants and randomly divide them into two separate groups. If the randomization is successful then these two groups should be the same in all respects, both measured confounders and unmeasured factors. The intervention is then implemented in one group and not the other and comparisons of intervention efficacy between the two groups are analysed. Theoretically, the only difference between the two groups through the entire study is the intervention. An excellent example is the intervention of a new medication to treat a specific disease among a group of patients. This randomization process is arguably the largest strength of an RCT ( 26 – 28 ). Additional methodological elements are utilized among RCTs to further strengthen the causal implication of the intervention’s impact. These include allocation concealment, blinding, measuring compliance, controlling for co-interventions, measuring dropout, analysing results by intention to treat, and assessing each treatment arm at the same time point in the same manner.
Crossover randomized controlled trial study design
A crossover RCT is a type of interventional study design where study participants intentionally “crossover” to the other treatment arm. This should not be confused with the observational case-crossover design. A crossover RCT begins the same as a traditional RCT, however, after the end of the first treatment phase, each participant is re-allocated to the other treatment arm. There is often a wash-out period in between treatment periods. This design has many strengths, including demonstrating reversibility, compensating for unsuccessful randomization, and improving study efficiency by not using time to recruit subjects.
Allocation concealment theoretically guarantees that the implementation of the randomization is free from bias. This is done by ensuring that the randomization scheme is concealed from all individuals involved ( 26 – 30 ). A third party who is not involved in the treatment or assessment of the trial creates the randomization schema and study participants are randomized according to that schema. By concealing the schema, there is a minimization of potential deviation from that randomization, either consciously or otherwise by the participant, researcher, provider, or assessor. The traditional method of allocation concealment relies upon sequentially numbered opaque envelopes with the treatment allocation inside. These envelopes are generated before the study begins using the selected randomization scheme. Participants are then allocated to the specific intervention arm in the pre-determined order dictated by the schema. If allocation concealment is not utilized, there is the possibility of selective enrolment into an intervention arm, potentially with the outcome of biased results.
Blinding in an RCT is withholding the treatment arm from individuals involved in the study. This can be done through use of placebo pills, deactivated treatment modalities, or sham therapy. Sham therapy is a comparison procedure or treatment which is identical to the investigational intervention except it omits a key therapeutic element, thus rendering the treatment ineffective. An example is a sham cortisone injection, where saline solution of the same volume is injected instead of cortisone. This helps ensure that patients do not know if they are receiving the active or control treatment. The process of blinding is utilized to help ensure equal treatment of the different groups, therefore continuing to isolate the difference in outcome between groups to only the intervention being administered ( 28 – 31 ). Blinding within an RCT includes patient blinding, provider blinding, or assessor blinding. In some situations it is difficult or impossible to blind one or more of the parties involved, but an ideal study would have all parties blinded until the end of the study ( 26 – 28 , 31 , 32 ).
Compliance is the degree of how well study participants adhere to the prescribed intervention. Compliance or non-compliance to the intervention can have a significant impact on the results of the study ( 26 – 29 ). If there is a differentiation in the compliance between intervention arms, that differential can mask true differences, or erroneously conclude that there are differences between the groups when one does not exist. The measurement of compliance in studies addresses the potential for differences observed in intervention arms due to intervention adherence, and can allow for partial control of differences either through post hoc stratification or statistical adjustment.
Co-interventions, interventions that impact the outcome other than the primary intervention of the study, can also allow for erroneous conclusions in clinical trials ( 26 – 28 ). If there are differences between treatment arms in the amount or type of additional therapeutic elements then the study conclusions may be incorrect ( 29 ). For example, if a placebo treatment arm utilizes more over-the-counter medication than the experimental treatment arm, both treatment arms may have the same therapeutic improvement and show no effect of the experimental treatment. However, the placebo arm improvement is due to the over-the-counter medication and if that was prohibited, there may be a therapeutic difference between the two treatment arms. The exclusion or tracking and statistical adjustment of co-interventions serves to strengthen an RCT by minimizing this potential effect.
Participants drop out of a study for multiple reasons, but if there are differential dropout rates between intervention arms or high overall dropout rates, there may be biased data or erroneous study conclusions ( 26 – 28 ). A commonly accepted dropout rate is 20% however, studies with dropout rates below 20% may have erroneous conclusions ( 29 ). Common methods for minimizing dropout include incentivizing study participation or short study duration, however, these may also lead to lack of generalizability or validity.
Intention-to-treat (ITT) analysis is a method of analysis that quantitatively addresses deviations from random allocation ( 26 – 28 ). This method analyses individuals based on their allocated intervention, regardless of whether or not that intervention was actually received due to protocol deviations, compliance concerns or subsequent withdrawal. By maintaining individuals in their allocated intervention for analyses, the benefits of randomization will be captured ( 18 , 26 – 29 ). If analysis of actual treatment is solely relied upon, then some of the theoretical benefits of randomization may be lost. This analysis method relies on complete data. There are different approaches regarding the handling of missing data and no consensus has been put forth in the literature. Common approaches are imputation or carrying forward the last observed data from individuals to address issues of missing data ( 18 , 19 ).
Assessment timing can play an important role in the impact of interventions, particularly if intervention effects are acute and short lived ( 26 – 29 , 33 ). The specific timing of assessments are unique to each intervention, however, studies that allow for meaningfully different timing of assessments are subject to erroneous results. For example, if assessments occur differentially after an injection of a particularly fast acting, short-lived medication the difference observed between intervention arms may be due to a higher proportion of participants in one intervention arm being assessed hours after the intervention instead of minutes. By tracking differences in assessment times, researchers can address the potential scope of this problem, and try to address it using statistical or other methods ( 26 – 28 , 33 ).
Randomized controlled trials are the principle method for improving treatment of disease, and there are some standardized methods for grading RCTs, and subsequently creating best practice guidelines ( 29 , 34 – 36 ). Much of the current practice of medicine lacks moderate or high quality RCTs to address what treatment methods have demonstrated efficacy and much of the best practice guidelines remains based on consensus from experts ( 28 , 37 ). The reliance on high quality methodology in all types of studies will allow for continued improvement in the assessment of causal factors for health outcomes and the treatment of diseases.
Standards of research and reporting
There are many published standards for the design, execution and reporting of biomedical research, which can be found in Table 3 . The purpose and content of these standards and guidelines are to improve the quality of biomedical research which will result in providing sound conclusions to base medical decision making upon. There are published standards for categories of study designs such as observational studies (e.g. STROBE), interventional studies (e.g. CONSORT), diagnostic studies (e.g. STARD, QUADAS), systematic reviews and meta-analyses (e.g. PRISMA ), as well as others. The aim of these standards and guideline are to systematize and elevate the quality of biomedical research design, execution, and reporting.
Published standard for study design and reporting.
Consolidated Standards Of Reporting Trials (CONSORT, www.consort-statement.org ) are interventional study standards, a 25 item checklist and flowchart specifically designed for RCTs to standardize reporting of key elements including design, analysis and interpretation of the RCT.
Strengthening the Reporting of Observational studies in Epidemiology (STROBE, www.strobe-statement.org ) is a collection of guidelines specifically for standardization and improvement of the reporting of observational epidemiological research. There are specific subsets of the STROBE statement including molecular epidemiology (STROBE-ME), infectious diseases (STROBE-ID) and genetic association studies (STREGA).
Standards for Reporting Studies of Diagnos tic Accuracy (STARD, www.stard-statement.org ) is a 25 element checklist and flow diagram specifically designed for the reporting of diagnostic accuracy studies.
Quality assessment of diagnostic accuracy studies (QUADAS, www.bris.ac.uk/quadas ) is a quality assessment of diagnostic accuracy studies.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, www.prisma-statement.org ) is a 27 element checklist and multiphase flow diagram to improve quality of reporting systematic reviews and meta-analyses. It replaces the QUOROM statement.
Consolidated criteria for reporting qualitative research (COREQ) is a 32 element checklist designed for reporting of qualitative data from interviews and focus groups.
Statistical Analyses and Methods in the Published Literature (SAMPL) is a guideline for statistical methods and analyses of all types of biomedical research.
Consensus-based Clinical Case Reporting Guideline Development (CARE, www.carestatement.org ) is a checklist comprised of 13 elements and is designed only for case reports.
Standards for Quality Improvement Reporting Excellence (SQUIRE, www.squire-statement.org ) are publication guidelines comprised of 19 elements, for authors aimed at quality improvement in health care reporting.
Consolidated Health Economic Evaluation Reporting Standards (CHEERS) is a 24 element checklist of reporting practices for economic evaluations of interventional studies.
Enhancing transparency in reporting the synthesis of qualitative research (ENTREQ) is a guideline specifically for standardizing and improving the reporting of qualitative biomedical research.
When designing or evaluating a study it may be helpful to review the applicable standards prior to executing and publishing the study. All published standards and guidelines are available on the web, and are updated based on current best practices as biomedical research evolves. Additionally, there is a network called “Enhancing the quality and transparency of health research” (EQUATOR, www.equator-network.org ) , which has guidelines and checklists for all standards reported in Table 3 and is continually updated with new study design or specialty specific standards.
The appropriate selection of a study design is only one element in successful research. The selection of a study design should incorporate consideration of costs, access to cases, identification of the exposure, the epidemiologic measures that are required, and the level of evidence that is currently published regarding the specific exposure-outcome relationship that is being assessed. Reviewing appropriate published standards when designing a study can substantially strengthen the execution and interpretation of study results.
Potential conflict of interest
None declared.
- 1. Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Wolters Kluwer Health; 2008. [ Google Scholar ]
- 2. Rothman KJ. Epidemiology: an introduction. Oxford University Press; 2012. [ Google Scholar ]
- 3. Szklo M, Nieto FJ. Epidemiology. Jones & Bartlett Publishers; 2012. [ Google Scholar ]
- 4. Nelson KE, Williams CM. Infectious disease epidemiology. Jones & Bartlett Publishers; 2013. [ Google Scholar ]
- 5. Ahrens W, Pigeot I. Handbook of epidemiology. Springer; New York: 2005. [ Google Scholar ]
- 6. Kelsey JL. Methods in observational epidemiology. 2 Ed. Oxford University Press; 1996. [ Google Scholar ]
- 7. MacMahon B, Pugh TF. Epidemiology: principles and methods. Epidemiology: principles and methods. 1970.
- 8. Checkoway H, Pearce NE, Kriebel DL. Research methods in occupational epidemiology. Oxford University Press; 2004. http://dx.doi.org/10.1093/acprof:oso/9780195092424.001.0001 . [ Google Scholar ]
- 9. Breslow NE. Case-control studies Handbook of Epidemiology. Springer; 2005. pp. 287–319. http://dx.doi.org/10.1007/978-3-540-26577-1_7 . [ Google Scholar ]
- 10. Friis RH, Sellers TA. Epidemiology for public health practice. Jones & Bartlett Publishers; 2013. [ Google Scholar ]
- 11. Aschengrau A, Seage G. Essentials of epidemiology in public health. Jones & Bartlett Publishers; 2008. [ Google Scholar ]
- 12. Woodward M. Epidemiology: study design and data analysis. CRC Press; 1999. [ Google Scholar ]
- 13. Haynes RB. Clinical epidemiology: how to do clinical practice research. LWW; 2012. [ Google Scholar ]
- 14. Petitti DB. Meta-analysis, decision analysis, and cost-effectiveness analysis: methods for quantitative synthesis in medicine. Oxford University Press; 1999. http://dx.doi.org/10.1093/acprof:oso/9780195133646.001.0001 . [ Google Scholar ]
- 15. Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. J. Wiley; 2000. [ Google Scholar ]
- 16. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Hegmann KT, Thiese MS, Oostema S, Melhorn JM. Causal Associations and Determination of Work Relatedness Guides to the evaluation of disease and injury causation. 2nd ed. Chicago: American Medical Association; 2013. pp. 105–14. [ Google Scholar ]
- 18. Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:670–4. doi: 10.1136/bmj.319.7211.670. http://dx.doi.org/10.1136/bmj.319.7211.670 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Frangakis CE, Rubin DB. Addressing complications of intention-to-treat analysis in the combined presence of all-or-none treatment-noncompliance and subsequent missing outcomes. Biometrika. 1999;86:365–79. http://dx.doi.org/10.1093/biomet/86.2.365 . [ Google Scholar ]
- 20. Monson RR. Analysis of relative survival and proportional mortality. Comput Biomed Res. 1974;7:325–32. doi: 10.1016/0010-4809(74)90010-x. http://dx.doi.org/10.1016/0010-4809(74)90010-X . [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Sheth T, Nair C, Nargundkar M, Anand S, Yusuf S. Cardiovascular and cancer mortality among Canadians of European, south Asian and Chinese origin from 1979 to 1993 an analysis of 1.2 million deaths. CMAJ. 1999;161:132–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Stolley PD. Investigating disease patterns: the science of epidemiology. 1995:x-242. [ Google Scholar ]
- 23. Schlesselman JJ, Schneiderman MA. Case control studies: design, conduct, analysis. Journal of Occupational and Environmental Medicine. 1982;24:879. [ Google Scholar ]
- 24. Yin RK. Case study research: Design and methods. Sage; 2009. [ Google Scholar ]
- 25. Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies: II. Types of controls. Am J Epidemiol. 135:1029–41. doi: 10.1093/oxfordjournals.aje.a116397. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Machin D, Fayers P. Randomized clinical trials: design, practice and reporting. Wiley. com; 2010. [ Google Scholar ]
- 27. Chow SC, Liu JP. Design and analysis of clinical trials: concepts and methodologies. Wiley. com; 2008. [ Google Scholar ]
- 28. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol. 2001;1:2. doi: 10.1186/1471-2288-1-2. http://dx.doi.org/10.1186/1471-2288-1-2 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Melhorn JM HK. Methodology. In: Melhorn JM AWI, editor. Guides to the Evaluation of Disease and Injury Causation. AMA Press; 2008. [ Google Scholar ]
- 30. Devereaux P, Manns BJ, Ghali WA, Quan H, Lacchetti C, Montori VM, et al. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. JAMA. 2001;285:2000–3. doi: 10.1001/jama.285.15.2000. http://dx.doi.org/10.1001/jama.285.15.2000 . [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. http://dx.doi.org/10.1016/0197-2456-(95)00134-4 . [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Devereaux P, Choi PT-L, El-Dika S, Bhandari M, Montori VM, Schünemann HJ, et al. An observational study found that authors of randomized controlled trials frequently use concealment of randomization and blinding, despite the failure to report these methods. J Clinical Epidemiol. 2004;57:1232–6. doi: 10.1016/j.jclinepi.2004.03.017. http://dx.doi.org/10.1016/j.jclinepi.2004.03.017 . [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Stevens A, Abrams K, Brazier J, Fitzpatrick R, Lilford R. The advanced handbook of methods in evidence based healthcare. Sage; 2001. [ Google Scholar ]
- 34. Hegmann K. In: Occupational medicine practice guidelines. Evaluation and management of common health problems and functional recovery in workers. 3rd ed. KT H, editor. Elk Grove Village (IL): American College of Occupational and Environmental Medicine (ACOEM); 2011. [ Google Scholar ]
- 35. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–E42. doi: 10.1503/cmaj.090449. http://dx.doi.org/10.1503/cmaj.090449 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Klazinga N. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project [AGREE] Qual Saf Health Care. 2003;12:18–23. doi: 10.1136/qhc.12.1.18. http://dx.doi.org/10.1136/qhc.12.1.18 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Harris JS, Sinnott PL, Holland JP, Ording J, Turkelson C, Weiss M, et al. Methodology to update the practice recommendations in the American College of Occupational and Environmental Medicine’s Occupational Medicine Practice Guidelines. J Occup Environ Med. 2008;50:282–95. doi: 10.1097/JOM.0b013e3181651613. http://dx.doi.org/10.1097/JOM.0b013e3181651613 . [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (113.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Experimental Studies
- Reference work entry
- Cite this reference work entry

- Sandra Šipetić Grujičić 2
1253 Accesses
7 Altmetric
Intervention studies
Experimental study is “study in which conditions are under the direct control of the investigator” (Last 2001 ). It is employed to test the efficacy of a preventive or therapeutic measure.
Experimental studies can provide the strongest evidence about the existence of a cause-effect relationship .
Basic Characteristics
Types of experimental studies.
There are two different types of experimental studies: therapeutic and prevention studies (Webb et al. 2005 ).
In therapeutic studies ( clinical trials ), different medicines or medical procedures for a given disease are compared in a clinical setting.
Trials that are conducted on healthy or apparently healthy individuals with the aim of preventing future morbidity or mortality are called preventive studies . Preventive studies include community study , in which the intervention is applied to groups, and field study , in which the intervention is applied to healthy individuals at usual or high risk of...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Bhopal R (2002) Concepts of epidemiology. An integrated introduction to the ideas, theories, principles, and methods of epidemiology. Oxford University Press, Oxford
Google Scholar
Dawson B, Trapp R (2001) Basic and clinical biostatistics, 3rd edn. Lange Medical Books/McGraw-Hill, New York
Fraceschi S, Plummer M (2005) Intervention trials. In: Ahrens W, Pigeot I (eds) Handbook of epidemiology. Springer, Berlin, pp 345–370
Friedman LM, Schron EB (2002) Methodology of intervention trials in individuals. In: Detels R, McEwen J, Beaglehole R, Tanaka H (eds) Oxford Textbook of Public Health, 4th edn. Oxford University Press, New York, pp 569–581
Gordis L (2004) Epidemiology, 3rd edn. Elsevier Saunders, Philadelphia
Last J (2001) A dictionary of epidemiology, 4th edn. Oxford University Press, New York
Webb P, Bain C, Pirozzo S (2005) Essential epidemiology: an introduction for students and health professionals. Cambridge University Press, Cambridge
Download references
Author information
Authors and affiliations.
Institute of Epidemiology, School of Medicine, University of Belgrade, Belgrade, Serbia
Sandra Šipetić Grujičić
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Network EUROlifestyle Research Association Public Health Saxony-Saxony Anhalt e.V. Medical Faculty, University of Technology, Fiedlerstr. 27, 01307, Dresden, Germany
Wilhelm Kirch ( Professor Dr. Dr. ) ( Professor Dr. Dr. )
Rights and permissions
Reprints and permissions
Copyright information
© 2008 Springer-Verlag
About this entry
Cite this entry.
Šipetić Grujičić, S. (2008). Experimental Studies . In: Kirch, W. (eds) Encyclopedia of Public Health. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-5614-7_1092
Download citation
DOI : https://doi.org/10.1007/978-1-4020-5614-7_1092
Publisher Name : Springer, Dordrecht
Print ISBN : 978-1-4020-5613-0
Online ISBN : 978-1-4020-5614-7
eBook Packages : Medicine Reference Module Medicine
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Observational vs. Experimental Study: A Comprehensive Guide
Explore the fundamental disparities between experimental and observational studies in this comprehensive guide by Santos Research Center, Corp. Uncover concepts such as control group, random sample, cohort studies, response variable, and explanatory variable that shape the foundation of these methodologies. Discover the significance of randomized controlled trials and case control studies, examining causal relationships and the role of dependent variables and independent variables in research designs.
This enlightening exploration also delves into the meticulous scientific study process, involving survey members, systematic reviews, and statistical analyses. Investigate the careful balance of control group and treatment group dynamics, highlighting how researchers meticulously assign variables and analyze statistical patterns to discern meaningful insights. From dissecting issues like lung cancer to understanding sleep patterns, this guide emphasizes the precision of controlled experiments and controlled trials, where variables are isolated and scrutinized, paving the way for a deeper comprehension of the world through empirical research.
Introduction to Observational and Experimental Studies
These two studies are the cornerstones of scientific inquiry, each offering a distinct approach to unraveling the mysteries of the natural world.
Observational studies allow us to observe, document, and gather data without direct intervention. They provide a means to explore real-world scenarios and trends, making them valuable when manipulating variables is not feasible or ethical. From surveys to meticulous observations, these studies shed light on existing conditions and relationships.
Experimental studies , in contrast, put researchers in the driver's seat. They involve the deliberate manipulation of variables to understand their impact on specific outcomes. By controlling the conditions, experimental studies establish causal relationships, answering questions of causality with precision. This approach is pivotal for hypothesis testing and informed decision-making.
At Santos Research Center, Corp., we recognize the importance of both observational and experimental studies. We employ these methodologies in our diverse research projects to ensure the highest quality of scientific investigation and to answer a wide range of research questions.
Observational Studies: A Closer Look
In our exploration of research methodologies, let's zoom in on observational research studies—an essential facet of scientific inquiry that we at Santos Research Center, Corp., expertly employ in our diverse research projects.
What is an Observational Study?
Observational research studies involve the passive observation of subjects without any intervention or manipulation by researchers. These studies are designed to scrutinize the relationships between variables and test subjects, uncover patterns, and draw conclusions grounded in real-world data.
Researchers refrain from interfering with the natural course of events in controlled experiment. Instead, they meticulously gather data by keenly observing and documenting information about the test subjects and their surroundings. This approach permits the examination of variables that cannot be ethically or feasibly manipulated, making it particularly valuable in certain research scenarios.
Types of Observational Studies
Now, let's delve into the various forms that observational studies can take, each with its distinct characteristics and applications.
Cohort Studies: A cohort study is a type of observational study that entails tracking one group of individuals over an extended period. Its primary goal is to identify potential causes or risk factors for specific outcomes or treatment group. Cohort studies provide valuable insights into the development of conditions or diseases and the factors that influence them.
Case-Control Studies: Case-control studies, on the other hand, involve the comparison of individuals with a particular condition or outcome to those without it (the control group). These studies aim to discern potential causal factors or associations that may have contributed to the development of the condition under investigation.
Cross-Sectional Studies: Cross-sectional studies take a snapshot of a diverse group of individuals at a single point in time. By collecting data from this snapshot, researchers gain insights into the prevalence of a specific condition or the relationships between variables at that precise moment. Cross-sectional studies are often used to assess the health status of the different groups within a population or explore the interplay between various factors.
Advantages and Limitations of Observational Studies
Observational studies, as we've explored, are a vital pillar of scientific research, offering unique insights into real-world phenomena. In this section, we will dissect the advantages and limitations that characterize these studies, shedding light on the intricacies that researchers grapple with when employing this methodology.
Advantages: One of the paramount advantages of observational studies lies in their utilization of real-world data. Unlike controlled experiments that operate in artificial settings, observational studies embrace the complexities of the natural world. This approach enables researchers to capture genuine behaviors, patterns, and occurrences as they unfold. As a result, the data collected reflects the intricacies of real-life scenarios, making it highly relevant and applicable to diverse settings and populations.
Moreover, in a randomized controlled trial, researchers looked to randomly assign participants to a group. Observational studies excel in their capacity to examine long-term trends. By observing one group of subjects over extended periods, research scientists gain the ability to track developments, trends, and shifts in behavior or outcomes. This longitudinal perspective is invaluable when studying phenomena that evolve gradually, such as chronic diseases, societal changes, or environmental shifts. It allows for the detection of subtle nuances that may be missed in shorter-term investigations.
Limitations: However, like any research methodology, observational studies are not without their limitations. One significant challenge of statistical study lies in the potential for biases. Since researchers do not intervene in the subjects' experiences, various biases can creep into the data collection process. These biases may arise from participant self-reporting, observer bias, or selection bias in random sample, among others. Careful design and rigorous data analysis are crucial for mitigating these biases.
Another limitation is the presence of confounding variables. In observational studies, it can be challenging to isolate the effect of a specific variable from the myriad of other factors at play. These confounding variables can obscure the true relationship between the variables of interest, making it difficult to establish causation definitively. Research scientists must employ statistical techniques to control for or adjust these confounding variables.
Additionally, observational studies face constraints in their ability to establish causation. While they can identify associations and correlations between variables, they cannot prove causality or causal relationship. Establishing causation typically requires controlled experiments where researchers can manipulate independent variables systematically. In observational studies, researchers can only infer potential causation based on the observed associations.
Experimental Studies: Delving Deeper
In the intricate landscape of scientific research, we now turn our gaze toward experimental studies—a dynamic and powerful method that Santos Research Center, Corp. skillfully employs in our pursuit of knowledge.
What is an Experimental Study?
While some studies observe and gather data passively, experimental studies take a more proactive approach. Here, researchers actively introduce an intervention or treatment to an experiment group study its effects on one or more variables. This methodology empowers researchers to manipulate independent variables deliberately and examine their direct impact on dependent variables.
Experimental research are distinguished by their exceptional ability to establish cause-and-effect relationships. This invaluable characteristic allows researchers to unlock the mysteries of how one variable influences another, offering profound insights into the scientific questions at hand. Within the controlled environment of an experimental study, researchers can systematically test hypotheses, shedding light on complex phenomena.
Key Features of Experimental Studies
Central to statistical analysis, the rigor and reliability of experimental studies are several key features that ensure the validity of their findings.
Randomized Controlled Trials: Randomization is a critical element in experimental studies, as it ensures that subjects are assigned to groups in a random assignment. This randomly assigned allocation minimizes the risk of unintentional biases and confounding variables, strengthening the credibility of the study's outcomes.
Control Groups: Control groups play a pivotal role in experimental studies by serving as a baseline for comparison. They enable researchers to assess the true impact of the intervention being studied. By comparing the outcomes of the intervention group to those of survey members of the control group, researchers can discern whether the intervention caused the observed changes.
Blinding: Both single-blind and double-blind techniques are employed in experimental studies to prevent biases from influencing the study or controlled trial's outcomes. Single-blind studies keep either the subjects or the researchers unaware of certain aspects of the study, while double-blind studies extend this blindness to both parties, enhancing the objectivity of the study.
These key features work in concert to uphold the integrity and trustworthiness of the results generated through experimental studies.
Advantages and Limitations of Experimental Studies
As with any research methodology, this one comes with its unique set of advantages and limitations.
Advantages: These studies offer the distinct advantage of establishing causal relationships between two or more variables together. The controlled environment allows researchers to exert authority over variables, ensuring that changes in the dependent variable can be attributed to the independent variable. This meticulous control results in high-quality, reliable data that can significantly contribute to scientific knowledge.
Limitations: However, experimental ones are not without their challenges. They may raise ethical concerns, particularly when the interventions involve potential risks to subjects. Additionally, their controlled nature can limit their real-world applicability, as the conditions in experiments may not accurately mirror those in the natural world. Moreover, executing an experimental study in randomized controlled, often demands substantial resources, with other variables including time, funding, and personnel.
Observational vs Experimental: A Side-by-Side Comparison
Having previously examined observational and experimental studies individually, we now embark on a side-by-side comparison to illuminate the key distinctions and commonalities between these foundational research approaches.
Key Differences and Notable Similarities
Methodologies
- Observational Studies : Characterized by passive observation, where researchers collect data without direct intervention, allowing the natural course of events to unfold.
- Experimental Studies : Involve active intervention, where researchers deliberately manipulate variables to discern their impact on specific outcomes, ensuring control over the experimental conditions.
- Observational Studies : Designed to identify patterns, correlations, and associations within existing data, shedding light on relationships within real-world settings.
- Experimental Studies : Geared toward establishing causality by determining the cause-and-effect relationships between variables, often in controlled laboratory environments.
- Observational Studies : Yield real-world data, reflecting the complexities and nuances of natural phenomena.
- Experimental Studies : Generate controlled data, allowing for precise analysis and the establishment of clear causal connections.
Observational studies excel at exploring associations and uncovering patterns within the intricacies of real-world settings, while experimental studies shine as the gold standard for discerning cause-and-effect relationships through meticulous control and manipulation in controlled environments. Understanding these differences and similarities empowers researchers to choose the most appropriate method for their specific research objectives.
When to Use Which: Practical Applications
The decision to employ either observational or experimental studies hinges on the research objectives at hand and the available resources. Observational studies prove invaluable when variable manipulation is impractical or ethically challenging, making them ideal for delving into long-term trends and uncovering intricate associations between certain variables (response variable or explanatory variable). On the other hand, experimental studies emerge as indispensable tools when the aim is to definitively establish causation and methodically control variables.
At Santos Research Center, Corp., our approach to both scientific study and methodology is characterized by meticulous consideration of the specific research goals. We recognize that the quality of outcomes hinges on selecting the most appropriate method of research study. Our unwavering commitment to employing both observational and experimental research studies further underscores our dedication to advancing scientific knowledge across diverse domains.
Conclusion: The Synergy of Experimental and Observational Studies in Research
In conclusion, both observational and experimental studies are integral to scientific research, offering complementary approaches with unique strengths and limitations. At Santos Research Center, Corp., we leverage these methodologies to contribute meaningfully to the scientific community.
Explore our projects and initiatives at Santos Research Center, Corp. by visiting our website or contacting us at (813) 249-9100, where our unwavering commitment to rigorous research practices and advancing scientific knowledge awaits.
Recent Posts
Join an Alzheimer's clinical trial at Santos Research Center. Discover treatment options, receive expert care, and help advance research. Apply for our paid trial today!
Discover the causes and health risks of obesity, plus explore new clinical trials at Santos Research Center offering innovative treatment options.
Learn about Bipolar I Disorder symptoms, treatments, and clinical trials at Santos Research Center. Explore new treatment options - join our trial today!
At Santos Research Center, a medical research facility dedicated to advancing TBI treatments, we emphasize the importance of tailored rehabilitation...
Learn about COVID-19 rebound after Paxlovid, its symptoms, causes, and management strategies. Join our study at Santos Research Center. Apply now!
Learn everything about Respiratory Syncytial Virus (RSV), from symptoms and diagnosis to treatment and prevention. Stay informed and protect your health with...
Discover key insights on Alzheimer's disease, including symptoms, stages, and care tips. Learn how to manage the condition and find out how you can...
Santos Research Center, Corp. is a research facility conducting paid clinical trials, in partnership with major pharmaceutical companies & CROs. We work with patients from across the Tampa Bay area.
Contact Details
Navigation menu.

IMAGES
VIDEO
COMMENTS
Experimental study design. The basic concept of experimental study design is to study the effect of an intervention. In this study design, the risk factor/exposure of interest/treatment is controlled by the investigator. Therefore, these are hypothesis testing studies and can provide the most convincing demonstration of evidence for causality.
Mar 7, 2024 · Originality/value This is the first study to review common mistakes in experimental research in hospitality research and to recommend some remedies. The findings of this study can contribute to ...
The main application of experimental studies, however, is in evaluating therapeutic interventions by randomised controlled trials. Randomised controlled trials At the outset of a randomised controlled trial the criteria for entry to the study sample must be specified (for example, in terms of age, sex, diagnosis, etc).
Jul 21, 2023 · Based on the methods used to collect data in experimental studies, the experimental research designs are of three primary types: 1. Pre-experimental Research Design. A research study could conduct pre-experimental research design when a group or many groups are under observation after implementing factors of cause and effect of the research.
Mar 26, 2024 · Select an Experimental Design Type. Choose a design type that aligns with your research question, hypothesis, and available resources. For example, an RCT for a medical study or a factorial design for complex interactions. Establish Experimental and Control Groups. Randomly assign participants to experimental or control groups.
Jan 1, 2022 · Different study designs are needed for the description of aging and for the analysis of the explanatory mechanisms that cause age-associated change. Scientists should use the most appropriate design to study their research questions. At a general level, observational (non-experimental) studies and experimental studies can be distinguished (Fig ...
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. In almost all experiments, at least one independent variable is varied and the effects on the dependent variable are investigated.
Interventional studies, also called experimental studies, are those where the researcher intercedes as part of the study design. Additionally, study designs may be classified by the role that time plays in the data collection, either retrospective or prospective.
Experimental study is “study in which conditions are under the direct control of the investigator” (Last 2001). It is employed to test the efficacy of a preventive or therapeutic measure. Experimental studies can provide the strongest evidence about the existence of a cause-effect relationship .
Dec 6, 2023 · Within the controlled environment of an experimental study, researchers can systematically test hypotheses, shedding light on complex phenomena. Key Features of Experimental Studies. Central to statistical analysis, the rigor and reliability of experimental studies are several key features that ensure the validity of their findings.